Current Research
Development of Bacillus subtilis as a bioproduction chassis
Bacillus subtilis is a GRAS, endotoxin-free platform that grows rapidly on inexpensive substrates and excels at secretion—ideal for extracellular enzymes and polymers. Its natural competence and mature genetic toolkit enable precise control of metabolism and expression for proteins and small molecules. At scale, however, B. subtilis is strongly oxygen-dependent: oxygen transfer (kLa) often limits productivity and forces high aeration/agitation, with penalties in foaming, shear, and energy cost. Overcoming this mass-transfer barrier is key to cost-effective, continuous operation.
We develop bioprocess concepts that improve oxygen transfer, raise cell density, and extend continuous run length—delivering higher space–time yields, lower aeration energy, and improved product quality. This positions B. subtilis as a competitive alternative to E. coli and yeast in oxygen-intensive bioprocesses.

Production of gamma-poly-glutamic acid (γ-PGA)
Escalating plastic waste is driving demand for biodegradable materials. γ-PGA— a natural glutamate polymer—typically comes as DL-mixtures with stereo-irregular backbones that form looser, heterogeneous networks. L-PGA, by contrast, is stereoregular (homochiral) and builds tighter, stronger gels suited to hydrogels, SAPs, and bioplastics—but present costs limit adoption.
We develop L-PGA production in Bacillus subtilis by integrating strain and process design to cut cost, raise productivity, and improve product quality/stability—targeting commodity-scale supply with higher space–time yields and lower energy use.
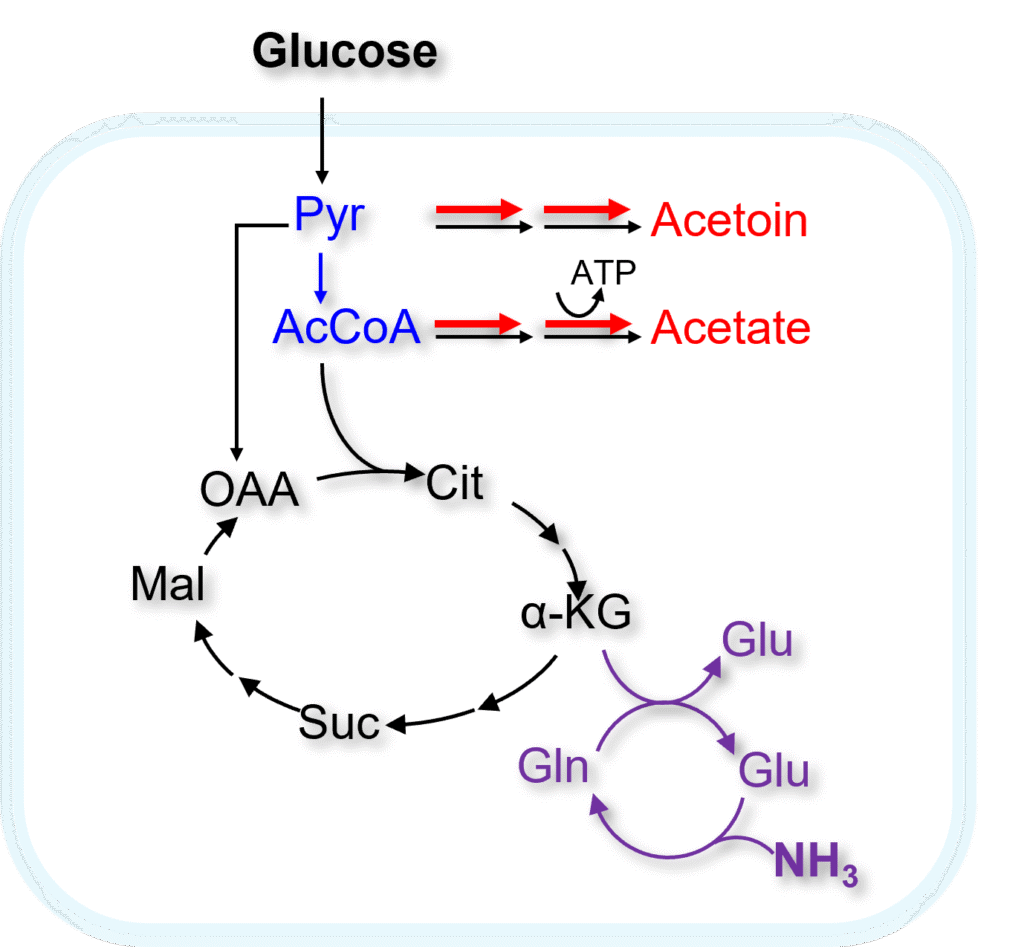
Central carbon flux biosensor
In Bacillus subtilis, fast glucose uptake plus catabolite repression can outpace oxidative capacity, pushing carbon into overflow byproducts (acetate, acetoin) and making performance sensitive to oxygen transfer.
Our project builds a central carbon flux biosensor that monitors the cell’s metabolic state and autonomously rebalances carbon flow. This system aims to create a self-tuning production chassis with higher yield and productivity under oxygen-limited conditions.

Accelerating evolution in Bacillus subtilis
Accelerated evolution (ALE) can significantly shorten natural adaptation timescales by creating genetic diversity and selecting under defined pressures. Beyond basic discovery, it particularly useful in bioproduction, where strains often face challenges such as high O₂ transfer, shear/foam, osmotic shifts, product toxicity, and tight redox and precursor constraints —traits that emerge too slowly by natural evolution.
We are establishing a genetic diversification and pooled-selection platform in B. subtilis to rapidly generate and screen variants under process-relevant regimes (high kLa, osmotic/solvent stress, pH shifts, continuous operation). The objective is to uncover genetic variants that improve robustness and productivity and then consolidate the best changes with precise edits.
